- Individuals can choose how they would like to administer insulin (pump, injection, or pen) based on a number or criteria. There is a common misconception that people have “mismanaged diabetes” to be recommended an insulin pump, and this is certainly not the case.
- The aim of someone with type 1 diabetes is to try to match food, physical activity (things that a person has some “control over”) and hormones, sleep patterns, stress, and many other components (that an individual has no “control” over) to maintain their glucose levels in a healthy range.
- Previously known as mature (or adult) onset diabetes, the reality is that today younger individuals are being increasingly diagnosed. There are many non-insulin treatments options available for type 2 diabetes, however there may come time when the body’s own insulin and the ability to maintain glucose levels in the healthy range are not equal, no matter how many tablets a person is taking. At this point external (exogenous) insulin is a treatment to maintain healthy blood glucose levels. People should never feel guilty about being prescribed insulin or feel like they have failed. Insulin should never be considered a punishment.
- Type 2 is a progressive health condition and healthcare professionals should be enabled to discuss this with people to support and reassure them. People may need to understand that the initial starting dose of insulin prescribed may not be their end dose. What determines the dose of insulin required will be determined by the effect on their blood glucose levels. People with diabetes should be recommended glucose monitoring in some form. This is especially important when first starting insulin or when adjusting dose. There is no way to determine the correct dose of insulin unless the health care professional can understand the effect of insulin on blood glucose levels from day to day.
- MedsChecks & Diabetes MedsChecks – Only people with type 2 diabetes qualify for Diabetes MedsChecks. People with type 1 diabetes qualify for MedsChecks only.
- Always remember to update the status with type 2 diabetes on the NDSS to Type 2 Insulin requiring so they can access insulin needles or syringes accordingly.
https://www.ndss.com.au/about-the-ndss/ndss-forms/ - If a person needs to start insulin urgently and does not have insulin requiring status, please fill out a manual needle form so they are able to receive syringes/needles on the day of insulin start. You are then able to send a copy of the insulin prescription and pen/syringe needle access form with contact details to Diabetes Australia in your state/territory via email (info@ndss.com.au) to have the person’s details updated. . https://www.ndss.com.au/about-the-ndss/ndss-forms/
Hypoglycaemia is the most common adverse effect of insulins. As with all insulins, particular caution including intensified blood glucose monitoring should be exercised in individuals who are at greater risk of clinically significant sequelae from hypoglycaemic episodes.
Ideally each person initiating insulin should see a Credentialled Diabetes Educator (CDE).
The role of a CDE is to ensure the person understands:
-Injection technique, with rotation of site. This is important to stop long term formation of lipodystrophy.
-Mechanism of action of insulin.
-Needle length – not one size fits all.
-Hypoglycaemia and sick day management.
If there is no time for the person to see a CDE before starting insulin, information on managing hypoglycaemia is available on the NDSS website:
https://www.ndss.com.au/living-with-diabetes/managing-diabetes/hypoglycaemia/
- MedsCheck or Diabetes MedsChek with counselling on side effect profile.
- Hypoglycaemia is part of the side effect profile of insulin and when occurring regularly, consider referral back to prescriber for adjustment of dose.
- Referral to see CDE for education on managing hypoglycaemia.
- Referral for blood glucose monitoring.
- Consideration should be given for glucagon to be prescribed with all insulin on a “just in case” basis.
- Ensure appropriate hypoglycaemia treatment is always carried- especially when participating in physical activity, driving, placed in the bedroom, when flying or going on holiday.
It is important for people using insulin to understand a new syringe/needle should be used for each injection.
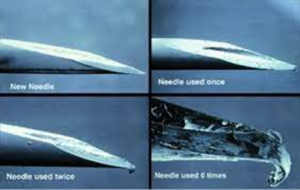
Injecting with a used needle or syringe can increase the risk of infection. Insulin has a large unpredictability component. Much depends on factors including:
-Skin temperature
-Insulin temperature
-Core body temperature (which influences blood flow),
-Site of injection and how much scar tissue there is),
-Hormones
-Sleep patterns
-Stress
Using blunt further adds to this variability.
Blunt needles can also cause scaring at the injection site, impacting insulin absorption long term. This is known as lipohypertropy.
- MedsCheck or Diabetes MedsCheck with counselling on needle & syringe use.
- Referral to healthcare team for injection technique.
- Counselling on disposal of all sharps. Disposal of sharps containers should also be handled appropriately.
Insulin not being used must be stored in the fridge (2-8 degrees). Remove new insulin pen/vial one hour before injecting. Injecting cold insulin is not dangerous but may cause discomfort.
Insulin that in use should be stored at room temperature for the entire time once opened. If not used within 28 days dispose as per state and territory instructions. Transfer of opened pens/vials between fridge and room temperature may cause instability of the insulin. Therefore, it is deemed better to store at room temperature (up to 25 degrees) for 28 days.
- MedsCheck or Diabetes MedsCheck with counselling on storage on insulin.
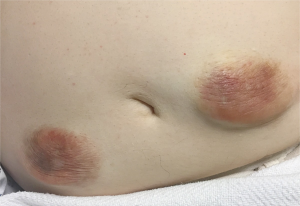
As with any insulin therapy, lipodystrophy may occur at the injection site and delay insulin absorption.
Other injection site reactions with insulin therapy include redness, pain, itching, hives, swelling and inflammation.
- MedsCheck or Diabetes MedsCheck with counselling on side effect profile
- Referral to healthcare team to establish correct injection technique.
Insulin administration may cause insulin antibodies to form. In rare cases, the presence of such insulin antibodies may necessitate adjustment of the insulin dose to correct a tendency to hyper- or hypoglycaemia.
- MedsCheck or Diabetes MedsCheck with referral to healthcare team for education if suspected.
Can include systems such as urticaria, chest tightness, dyspnoea, allergic dermatitis and pruritis. Severe cases of generalised allergy, including anaphylactic reaction, may be life-threatening and are uncommon.
- MedsCheck or Diabetes MedsCheck with referral to healthcare team if suspected.
- Ryzodeg is not a mixed insulin, instead it is a co-formulation.
- Ryzodeg Flexpen dose button does not extend when a dose is dialled on the pen.
- Ryzodeg co-formulation is 70% Degludec (Ultra long-acting analogue) & 30% Aspart (Rapid acting analogue). When injected into subcutaneous layer, Degludec forms distinct multi-hexamers that are absorbed slowly. Aspart analogue forms monomers and is rapidly absorbed. Each component of the formulation does not interact with the other.
- MedsCheck or Diabetes MedsCheck with counselling on Ryzodeg Flexpen dose button. When an individual is transferring from another insulin and is familiar with the dose button extending on an insulin pen device, advise the Ryzodeg Flexpen dose button does not extend when a dose is dialled on the pen. This has been designed to aid dexterity and to aid in a lower ejection force. It is often the case that an individual that has used a different insulin injection device may feel they are not receiving a dose or that the insulin pen is broken since they cannot physically see the top of the button where they are pressing moving (see picture where thumb is resting). The dose button will return to zero once the entire dose has been administered.
- Fiasp can be used for continuous subcutaneous insulin infusion (CSII) (insulin pumps). However, at the time of writing Fiasp was not approved for use in the Tslimx2 insulin pump.
- Statement from AMSL about Fiasp in the Tslim Insulin Pump
- “Tandem pumps and cartridges are only indicated for use with Novorapid and Humalog U-100 insulins. The safety and efficacy of other insulins, including Fiasp, have not been established for use with Tandem insulin pumps. Importantly, clinical studies using our automated insulin delivery algorithms were only conducted using Humalog and Novorapid U-100 insulins, which have different times of onset compared to Fiasp.”
- https://amsldiabetes.com.au/products/tslim-x2-insulin-pump/
- This is due to the fact there have been ongoing issues with occlusions (blockages) which may result in elevated glucose levels.
- MedsCheck with counselling on Fiasp not being approved for use in the Tslimx2 insulin pump.
- Referral back to healthcare team.
Statement from Sanofi about the use of Apidra insulin in insulin pumps
Please note, continuous subcutaneous insulin infusion(CSII) via an external pump is not an approved route of administration for Apidra. Sanofi does not recommend the use of Apidra in any manner inconsistent with that described in the Apidra Product Information
- MedsCheck with referral back to healthcare team.
Preparations containing cloudy insulins should be gently agitated by “rocking and rolling” between the hands before use to ensure that the insulin is uniformly distributed throughout the liquid. The injection should be given immediately thereafter.
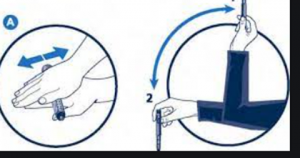
- MedsCheck or Diabetes MedsCheck with counselling on insulin preparation.
Occasionally the choice of insulin is not about how the insulin works but about what delivery system is most suitable for the person with diabetes (person centred care). Mixtard 30/70 and Protaphane insulin comes in a Innolet device (see below) that is designed for those who have low vision or problems with dexterity.
The dial is larger than that of the regular insulin pens and is also raised so individuals can feel when they are dialling up their insulin dose. The “foot” is also designed to sit on the skin while the injection is given allowing the needle to remain stable. This causes less bruising and irritation.
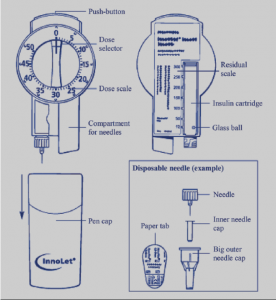
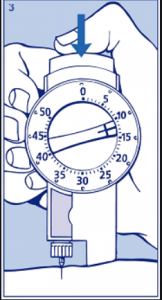
- MedsCheck or Diabetes MedsCheck with counselling on Innolet device.
- Referral to healthcare team for Innolet device education if required.
