Pharmacy/Access Point Product Information
Background
The Australian Government provides access to fully subsidised Continuous Glucose Monitoring (CGM) & Flash Glucose Monitoring products for eligible participants with diabetes through the National Diabetes Services Scheme (NDSS).
To access CGM & Flash Glucose Monitoring under the NDSS eligible participants must have the following:
- Type 1 Diabetes
- Concession/Pension card
- Paperwork filled and logged to NDSS by CDE or endocrinologist or physician who specializes in diabetes.
The choice of which specific device to be used is a decision of the authorised health professional in consultation with, and with the informed consent of, eligible participants and/or their carer.
Sensors & Transmitters
Continuous and Flash Glucose Monitoring products have firm product use limits that are based on use of the device as advised by the manufacturer. Eligible participants are allocated a defined number of sensors and transmitters each funded calendar year. Limits cannot be exceeded in any calendar year.
Once approved eligible participants can order allocated transmitter and sensors through community pharmacy acting as NDSS access points. Once ordered, sensors or transmitters will be available for collection at pharmacies/access points within 24-48 hours in most circumstances.
Sensor and transmitters are allocated per eligible participant and not to pharmacies/access points. Pre-ordering sensors and transmitters on behalf of eligible participants can impact eligible participant allocations if the following occurs:
- Eligible participants change their pharmacy/access point. Any sensors or transmitted pre-ordered by the pharmacy and not collected will reduce the total number available to eligible participants if they decide to change pharmacy/access point.
- Eligible participants are on holiday and want to access sensors and transmitters from another pharmacy/access point. This can impact eligible participant available allocations as they reach the end of the calendar year, a common holiday period.
There is no expectation for pharmacies to keep these NDSS products in stock as with blood glucose monitoring strips and needles for use with injectable medicine. Pharmacies purchasing and carrying additional inventory as a service to eligible participants may leave pharmacies out of pocket if the following occurs:
- Eligible participants change Pharmacy/Access point.
- Eligible participants in consultation with their healthcare professional change device.
Sensor & Transmitter Types
Dexcom
G6 transmitter

G6 sensor applicator sensor without transmitter attached
G5Transmitter

G5 Sensor applicator
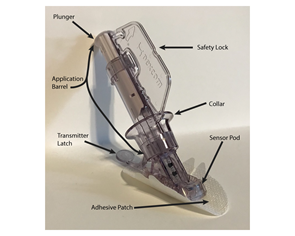
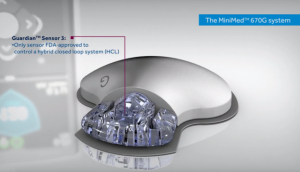
Medtronic
Sensor and Transmitter
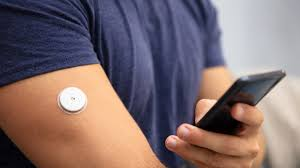
Abbott Libre Flash Glucose Monitoring
Abbott Libre Flash Glucose Monitoring is not classified as CGM since it does not send alarms to a third-party device like an insulin pump or smart phone. It does however provide a person with diabetes a significant amount of data when they scan their smart phone or reader over the sensor which is usually worn in their arm.
Eligible participants are also allocated a defined number of sensors under the NDSS funding each year. The same access issues arise as described above when pharmacies/access points pre-order sensors on behalf of eligible participants.
Eligible participants are also allocated a defined number of sensors under the NDSS funding each year. The same access issues arise as described above when pharmacies/access points pre-order sensors on behalf of eligible participants.
Abbott Libre Sensor
Sensor
Continuous & Flash Glucose Monitoring Product Faults
If there are any problems with Continuous & Flash monitoring Transmitter and Sensors, such as the sensor comes off or the battery wears out before its warranty period, the person with diabetes is encouraged to seek help from the manufacture who will replace it and not use their NDSS stock allocation.
There is no expectation for pharmacies to keep these NDSS products in stock as there is with blood glucose monitoring strips and needles for use with injectable medicine.
There is no expectation for pharmacies to keep these NDSS products in stock as there is with blood glucose monitoring strips and needles for use with injectable medicine.
