- Empagliflozin and metformin
Empagliflozin/ metformin is available in six different strength combinations.
- Empagliflozin/ metformin 5 mg/500 mg contains 5 mg empagliflozin and 500 mg metformin
- Empagliflozin/ metformin 5 mg/850 mg contains 5 mg empagliflozin and 850 mg metformin
- Empagliflozin/ metformin 5 mg/1000 mg contains 5 mg empagliflozin and 1000 mg metformin
- Empagliflozin/ metformin 12.5 mg/500 mg contains 12.5 mg empagliflozin and 500 mg metformin
- Empagliflozin/ metformin 12.5 mg/850 mg contains 12.5 mg empagliflozin and 850 mg metformin
- Empagliflozin/ metformin 12.5 mg/1000 mg contains 12.5 mg empagliflozin and 1000 mg metformin
- Empagliflozin/ metformin is indicated as an adjunct to healthy eating and physical activity to improve glycaemic management in adults with type 2 diabetes mellitus when treatment with both empagliflozin and metformin is appropriate.
- Empagliflozin is indicated in adults with type 2 diabetes mellitus and established cardiovascular disease to reduce the risk of cardiovascular death.
- To prevent cardiovascular deaths, empagliflozin should be used in conjunction with other measures to reduce cardiovascular risk in line with the current standard of care.
For the latest PBS indications for Empagliflozin/ metformin please see
https://www.pbs.gov.au/medicine/item/10626g-10627h-10633p-10639y-10640b-10649l-10650m-10677y
Life threatening lactic acidosis can occur due to accumulation of metformin. Risk factors include renal impairment, old age and the use of high doses of metformin above 2000mg per day.
- The recommended dose of empagliflozin/ metformin is one tablet twice daily.
- Adults with normal renal function (GFR ≥ 90mL/min) The dosage should be individualised on the basis of the person’s current regimen, effectiveness, and tolerability. The maximum recommended daily dose of empagliflozin/ metformin is 25 mg of empagliflozin and 2000 mg of metformin.
- Empagliflozin/ metformin should be given with meals to reduce the gastrointestinal undesirable effects associated with metformin.
- Treatment naïve individuals:
-The recommended starting dose is 5 mg/500 mg twice daily.
-If additional glycaemic management is required, adjust dosing based on effectiveness and tolerability while not exceeding the maximum recommended daily dose of 25 mg empagliflozin and 2000 mg metformin.
Individuals switching from separate tablets of empagliflozin and metformin:
-People switching from separate tablets of empagliflozin (10 mg or 25 mg total daily dose) and metformin to empagliflozin/ metformin, should receive the same daily dose of empagliflozin and metformin already being taken or the nearest therapeutically appropriate dose of metformin.
Individuals not adequately managed on the maximal tolerated dose of metformin alone or in combination with other products, including insulin:
-The recommended starting dose of empagliflozin/ metformin should provide empagliflozin 5 mg twice daily (10 mg total daily dose) and the dose of metformin like the dose already being taken.
In individuals tolerating a total daily dose of empagliflozin 10 mg, the dose can be increased to a total daily dose of empagliflozin 25 mg.
Combination use:
When empagliflozin/ metformin is used in combination with a sulfonylurea and/or insulin, a lower dose of sulfonylurea and/or insulin may be required to reduce the risk of hypoglycaemia.
Note: Diabetes Medscheck re hypoglycaemia and counselling for referral to appropriate health care professional for dose adjustment if required.
- Renal impairment:
No dose adjustment is recommended for those with mild renal impairment. Empagliflozin/ metformin is contraindicated for use in severe renal impairment (creatinine clearance <30 ml/min).
Renal function should be assessed before initiation of treatment with empagliflozin/ metformin and at least annually thereafter. In those at an increased risk of further progression of renal impairment and in the elderly, renal function should be assessed more frequently, e.g., every 3-6 months
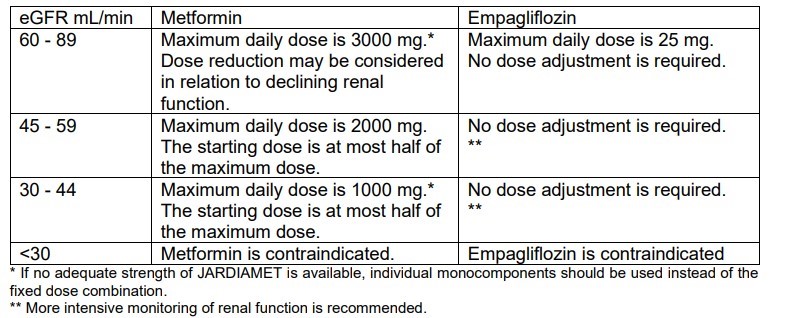
- Hepatic impairment:
Empagliflozin/ metformin is contraindicated in those with hepatic impairment due to the metformin component.
Individuals aged 75 years and older may be at an increased risk of volume depletion, therefore, empagliflozin/ metformin should be prescribed with caution. Therapeutic experience in people aged 85 years and older is limited. Initiation of treatment in this population is not recommended.
- population: does this have its own toggle??
- Empagliflozin/ metformin is not recommended for use in children below 18 years due to lack of data on safety and efficacy.
- Hypersensitivity to active ingredients empagliflozin and/or metformin hydrochloride or to any of the excipients
- Type 1 diabetes
- Any type of metabolic acidosis (such as lactic acidosis, ketoacidosis)
- Pre-coma due to diabetes
- Severe renal failure (creatinine clearance < 30mL/min or eGFR < 30 mL/min/1.73m2 which may also result from conditions such as cardiovascular collapse (shock), acute myocardial infarction, and septicaemia
- Acute conditions with the potential to alter renal function such as: dehydration, severe infection, shock, intravascular administration of iodinated contrast agents
- Acute or chronic disease which may cause tissue hypoxia such as: cardiac or respiratory failure, recent myocardial infarction, shock, pulmonary embolism, acute significant blood loss, sepsis, gangrene, pancreatitis
- During or immediately following surgery where insulin is essential, elective major surgery
- Hepatic impairment, acute alcohol intoxication, alcoholism (due to the metformin component)
- Lactation
- Empagliflozin/ metformin must be temporarily discontinued when undergoing radiologic studies involving intravascular administration of iodinated contrast materials because use of such products may result in acute alteration of renal function.
General:
Ertugliflozin / sitagliptin should not be used in those with type 1 diabetes mellitus or for the treatment of ketoacidosis.
- Sitagliptin
- Pancreatitis
-There have been reports of acute pancreatitis, including fatal and non-fatal haemorrhagic or necrotizing pancreatitis in individuals taking sitagliptin, a component of ertugliflozin / sitagliptin. Individuals should be informed of the characteristic symptoms of acute pancreatitis including persistent, severe abdominal pain. Resolution of pancreatitis has been observed after discontinuation of sitagliptin. If pancreatitis is suspected, ertugliflozin / sitagliptin and other potentially suspect medicinal products should be discontinued.
Note: Diabetes MedsCheck with counselling on side effect profile and referral to healthcare team as required.
- Hypersensitivity reactions:
-There have been post marketing reports of serious hypersensitivity reactions in those treated with sitagliptin. These reactions include anaphylaxis, angioedema, and exfoliative skin conditions including Stevens-Johnson syndrome. Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure. Onset of these reactions occurred within the first 3 months after initiation of treatment with sitagliptin, with some reports occurring after the first dose. If a hypersensitivity reaction is suspected, discontinue ertugliflozin / sitagliptin, assess for other potential causes for the event, and institute alternative treatment for diabetes.
- Arthralgia
-There have been post-marketing reports of joint pain, which may be severe, in people taking DPP-4 inhibitors. Onset of symptoms following initiation of treatment may be rapid or may occur after longer periods. Discontinuation of therapy should be considered in those who present with or experience an exacerbation of joint symptoms during treatment with DPP-4 inhibitors.
- Bullous pemphigoid:
-Post marketing cases of bullous pemphigoid requiring hospitalisation have been reported with DPP-4 inhibitor use. In reported cases, individuals typically recovered with topical or systemic immunosuppressive treatment and discontinuation of the DPP-4 inhibitor. Counsel individuals to report development of blisters or erosions while receiving ertugliflozin / sitagliptin. If bullous pemphigoid is suspected, ertugliflozin / sitagliptin should be discontinued and referral to a dermatologist should be considered for diagnosis and appropriate treatment.
- Ertugliflozin
- Hypotension/Volume depletion
-Ertugliflozin, can cause intravascular volume contraction that may sometimes manifest as symptomatic hypotension or acute transient changes in creatinine. Those with impaired renal function (eGFR less than 60 mL/min/1.73 m2), the elderly (≥65 years), or people on diuretics may be at increased risk for volume depletion or hypotension. Before initiating ertugliflozin / sitagliptin in individuals with one or more of these characteristics, intravascular volume status should be assessed, and people advised on the importance of adequate hydration. Monitor intravascular volume status in addition to blood pressure and renal function after initiating therapy. In case of conditions that may lead to fluid loss (e.g., gastrointestinal illness, heat stress or severe infections), careful monitoring of volume status (e.g., physical examination, blood pressure measurements, laboratory tests including haematocrit) and electrolytes is recommended for individuals receiving ertugliflozin / sitagliptin Temporary interruption of ertugliflozin / sitagliptin should be considered until the fluid loss is corrected.
Note: Diabetes Medscheck- Counsel on side effect profile, blood pressure monitoring in pharmacy with referral to appropriate health care professional if blood pressure is unstable.
- Ketoacidosis
-Ertugliflozin / sitagliptin should not be used for the treatment of ketoacidosis. Reports of ketoacidosis, a serious life-threatening condition requiring urgent hospitalisation, have been identified in clinical trials and post marketing surveillance in those receiving sodium glucose co-transporter-2 (SGLT2) inhibitors. Fatal cases of ketoacidosis have been reported in individuals taking SGLT2 inhibitors. Ertugliflozin / sitagliptin is not indicated for the treatment of people with type 1 diabetes mellitus. Individuals treated with ertugliflozin / sitagliptin who present with signs and symptoms consistent with severe metabolic acidosis should be promptly assessed for ketoacidosis regardless of presenting blood glucose levels, as ketoacidosis associated with SGLT2 inhibitors may be present even if blood glucose levels are less than 14 mmol/L. If ketoacidosis is suspected, ertugliflozin / sitagliptin should be discontinued, the person should be evaluated, and prompt treatment should be instituted. Treatment of ketoacidosis generally requires insulin, fluid, potassium, and carbohydrate replacement. Signs and symptoms of ketoacidosis may include excessive thirst, nausea, vomiting, abdominal pain, generalised malaise, and shortness of breath. Restarting SGLT2 inhibitor treatment in those with previous ketoacidosis while on SGLT2 inhibitor treatment is not recommended unless another clear precipitating factor is identified and resolved. Before initiating ertugliflozin / sitagliptin, consider factors in the history that may predispose to ketoacidosis. Factors that predispose patients to ketoacidosis include a low carbohydrate diet, dehydration, acute illness, surgery, a previous ketoacidosis, insulin dose reduction, malnourishment / reduced caloric intake or increased insulin requirements due to infections, insulin deficiency from any cause (including history of pancreatitis or pancreatic surgery, alcohol abuse). Ertugliflozin / sitagliptin should be used with caution in these individuals. Consider monitoring for ketoacidosis and temporarily discontinuing ertugliflozin / sitagliptin in clinical situations known to predispose to ketoacidosis.
Note: Diabetes MedsCheck with referral to healthcare team especially credentialled diabetes educator for counselling on monitoring of ketones and what to do should ketones present in elevated numbers.
- Surgery
-Treatment with ertugliflozin / sitagliptin should be ceased prior to major surgery. An increase in other glucose lowering agents may be required during this time. Those scheduled for non-urgent surgery who have not ceased ertugliflozin should be assessed and consideration should be given to postponing the procedure. Treatment with ertugliflozin / sitagliptin may be restarted once a person’s condition has stabilised and oral intake is normal.
Note: Diabetes MedsCheck with education on sick day management with referral to health care team.
- Genital infections
-Ertugliflozin increases the risk of genital infections. In trials with SGLT2 inhibitors, individuals with a history of genital infections and uncircumcised males were more likely to develop genital infections,
Note: Diabetes MedsCheck with education on side effect profile
- Necrotising fasciitis of the perineum:
-Post marketing cases of necrotising fasciitis of the perineum (also known as Fournier’s gangrene), a rare, but serious and life-threatening necrotising infection, have been reported in females and males with diabetes mellitus treated with SGLT2 inhibitors. Serious outcomes have included hospitalisation, multiple surgeries, and death. Those treated with ertugliflozin / sitagliptin who present with pain or tenderness, erythema, swelling in the genital or perineal area, fever, and malaise should be evaluated for necrotising fasciitis. If suspected, ertugliflozin / sitagliptin should be discontinued, and prompt treatment should be instituted (including broad-spectrum antibiotics and surgical debridement if necessary). Caution is advised in individuals at increased risk of genital infections including those with recurrent or pre-existing urogenital infections, obesity, immunosuppressed states, smoking, alcohol abuse, end-stage renal or liver failure, and HbA1c >10%.
Note: Diabetes MedsCheck with education on side effects profile.
Genital infection
Urinary tract infection
Taste disturbance
Lactic acidosis
Vitamin B12 deficiency
Dysuria
Volume depletion
Glomerular filtration rate decreased
Blood creatinine increased
Haematocrit increased
Gastrointestinal symptoms
- The results of bioequivalence studies in those without diabetes demonstrated that empagliflozin/metformin 5 mg/500 mg, 5 mg/850 mg, 5 mg/1000 mg, 12.5 mg/500 mg, 12.5 mg/850 mg, and 12.5 mg/1000 mg combination tablets are bioequivalent to co-administration of corresponding doses of empagliflozin and metformin as individual tablets.
- The observed effect of food on empagliflozin and metformin is not considered to be clinically relevant. However, as metformin is recommended to be given with meals, empagliflozin/ metformin is also proposed to be given with food. The following statements reflect the pharmacokinetic properties of the individual active substances of empagliflozin/ metformin.
After oral administration, empagliflozin was rapidly absorbed with peak plasma concentrations occurring at a median tmax 1.5 h post-dose. Overall exposure (AUCss) of empagliflozin over a 24-hour period with 5 mg administered twice daily was similar to 10 mg administered once daily.
Metformin
After an oral dose of metformin, tmax is reached in 2.5 hours. Absolute bioavailability of a 500 mg or 850 mg metformin hydrochloride tablet is approximately 50-60% in those without diabetes. After an oral dose, the non-absorbed fraction recovered in faeces was 20-30%. At the recommended metformin doses and dosing schedules, steady state plasma concentrations are reached within 24 to 48 hours
The apparent steady-state volume of distribution was estimated to be 73.8 L, based on a population pharmacokinetic analysis. Following administration of an oral [14C]-empagliflozin solution to people without diabetes, the red blood cell partitioning was approximately 36.8% and plasma protein binding was 86.2%.
Metformin
Plasma protein binding is negligible. Metformin e partitions into erythrocytes. The blood peak is lower than the plasma peak and appears at approximately the same time. The red blood cells most likely represent a secondary compartment of distribution. The mean volume of distribution (Vd) ranged between 63-276 L.
No major metabolites of empagliflozin were detected in human plasma and the most abundant metabolites were three glucuronide conjugates (2-O-, 3-O-, and 6-O-glucuronide). Systemic exposure of each metabolite was less than 10% of total drug-related material. In vitro studies suggested that the primary route of metabolism of empagliflozin in humans is glucuronidation by the uridine 5′-diphospho-glucuronosyltransferases.
Metformin
Metformin is excreted unchanged in the urine and does not undergo hepatic metabolism.
The apparent terminal elimination half-life of empagliflozin was estimated to be 12.4 h and apparent oral clearance was 10.6 L/h based on the population pharmacokinetic analysis. The inter-subject and residual variabilities for empagliflozin oral clearance were 39.1% and 35.8%, respectively.
Metformin
Renal clearance of metformin is >400 mL/min, indicating that metformin e is eliminated by glomerular filtration and tubular secretion. Following an oral dose, the apparent terminal elimination half-life is approximately 6.5 hours. When renal function is impaired, renal clearance is decreased in proportion to that of creatinine and thus the elimination half-life is prolonged, leading to increased levels of metformin hydrochloride in plasma.
For further information on empagliflozin/metformin please go to
https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2015-PI-02229-1
