Name of Medicine
Glucagon hydrochloride
Presentation
- GlucaGen HypoKit contains glucagon hydrochloride. The reconstituted solution contains glucagon 1 mg/mL and lactose monohydrate 107 mg/mL.
Key Practice Points
Therapeutic Indications:
- For the management of insulin requiring diabetes.
For the latest PBS indications for Protaphane please see
https://www.pbs.gov.au/pbs/search?term=protaphane&analyse=false&search-type=medicines
Dose:
- Protaphane is usually given once or twice daily. It can be given alone or mixed with short acting insulin.
- In intensive insulin therapy Protaphane can be used as basal insulin (evening and/or morning injection) with soluble insulin and given at meals.
- Protaphane should never be administered intravenously. It also should never be used in insulin infusion pump.
- Reconstitution of cloudy insulin: Preparations containing cloudy insulins should be gently agitated by “rocking and rolling” between the hands before use to ensure that the insulin is uniformly distributed throughout the liquid. The injection should be given immediately thereafter.
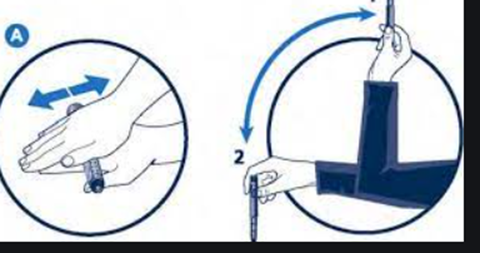
- Mixing with other insulins. Insulin preparations may be mixed in a syringe. The insulin mixture should be injected immediately after preparation. When short acting soluble insulin is mixed with longer acting insulin, the short acting insulin should be drawn into the syringe first to prevent contamination of the vial by the longer acting preparation.
- Renal impairment: Insulin requirements maybe diminished due to reduced insulin metabolism. Careful glucose monitoring and dose adjustments of insulin, including Protaphane, maybe necessary. Note: Diabetes MedsCheck with referral for annual cycle of care.
- Hepatic impairment: Insulin requirements may be diminished due to reduced capacity for gluconeogenesis and reduced insulin metabolism. Careful glucose monitoring and dose adjustments of insulin, including Protaphane, may be necessary.
Contraindications:
- Hypoglycaemia
- Hypersensitivity to Protaphane insulin or any of the excipients.
- Insulin suspensions should not be administered intravenously.
- Insulin suspensions are not suitable for the treatment of diabetic coma.
Elderly:
- Elderly: progressive deterioration of renal function may lead to steady decrease in insulin requirements. Careful glucose monitoring and dose adjustments of insulin, including Protaphane, maybe necessary in the elderly.
Precautions:
- Hyperglycaemia: Inadequate dosing or discontinuation of treatment, especially in type 1 diabetes, may lead to hyperglycaemia and ketoacidosis. The first symptoms of hyperglycaemia usually come on gradually over a period of hours or days. They include nausea, vomiting, drowsiness, flushed dry skin, dry mouth, increased urination, thirst and loss of appetite as well as acetone breath. In type 1 diabetes, untreated hyperglycaemic events lead to ketoacidosis which is potentially lethal. Note: Diabetes MedsCheck with counselling on hyperglycaemia and referral to healthcare for sick day management.
- Travelling with Protaphane: Before travelling between different time zones, an individual on Protaphane should be advised to consult their doctor or credentialled diabetes educator, since this may mean that the person may need to inject their insulin and eat their meals at different times. Note: Diabetes MedsCheck with counselling how to plan appropriately to avoid side effects, and referral to healthcare team for further education.
Adverse Effects:
- Hypoglycaemia: Hypoglycaemia is the most common adverse effect of insulins. As with all insulins, particular caution (including intensified blood glucose monitoring) should be exercised in individuals who are at greater risk of clinically significant sequelae from hypoglycaemic episodes. These include (but are not restricted to) change in the injection area improved insulin sensitivity (e.g., by removal of stress factors, weight loss); unaccustomed, increased, or prolonged physical activity. intercurrent illness (e.g., vomiting, diarrhoea); inadequate food intake; missed meals; and alcohol consumption. The prolonged effect of subcutaneous insulin Protaphane may delay recovery from hypoglycaemia. Note: Diabetes MedsCheck with counselling on side effect profile and hypoglycaemia is part of the side effect profile. Referral for blood glucose monitoring. If hypoglycaemia is happening regularly consider referral to healthcare team for adjustment of dose.
- Eyes: A marked change in glycaemic management may cause temporary visual impairment, due to temporary alteration in the turgidity and refractive index of the lens. As with all insulin regimens, intensification of insulin therapy with abrupt improvement in glycaemic management maybe associated with temporary visual impairment or worsening of retinopathy. However, long-term improved glycaemic management decreases the risk of progression of retinopathy. Note: Diabetes MedsCheck with counselling on insulin mode of action and referral to healthcare team when required.
- Injection site and allergic reactions. As with any insulin therapy, lipodystrophy may occur at the injection site and delay insulin absorption. Other injection site reactions with insulin therapy include redness, pain, itching, hives, swelling and inflammation. Note: Diabetes MedsCheck with counselling on side effect profile and referral to healthcare team to establish correct injection technique.
Pharmacokinetic Properties Summary
- Protaphane is an intermediate acting insulin preparation. Its hypoglycaemic effect after subcutaneous administration begins after approximately1.5 hours, is maximal between 4 and 12 hours, and lasts up to approximately24 hours.
